With the inclusion of the Human Papillomavirus (HPV) vaccine in the National Immunization Program. The province of Holguin is working to organize the start of the campaign. Scheduled for October 27th to December 27th of this year.
According to Dr. Geanela Cruz Ávila, director of the Provincial Center for Hygiene, Epidemiology, and Microbiology (CPHEM), “Holguin is ready to begin vaccination. The population of 4,875 nine-year-old girls who will be vaccinated has been defined.” As it is more effective before the onset of sexual activity, according to international recommendations.
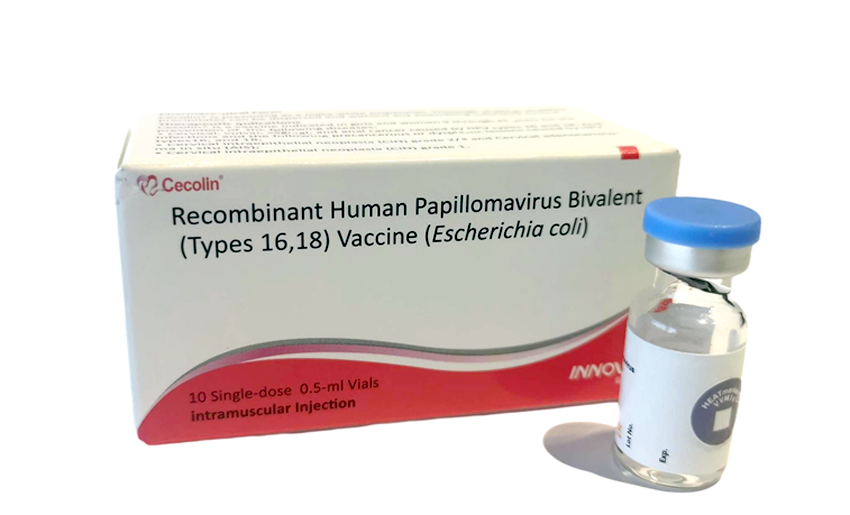
The CECOLIN bivalent vaccine produced by the Chinese company Innovax. Which has been prequalified by the World Health Organization (WHO), will be used for immunization.
Regarding the details of its composition, safety, efficacy, and route of administration. The health official explained that its purpose is to eliminate cervical cancer. Reducing the rate to less than four cases per 100,000 women per year.
She added that they are in the process of receiving and communicating it to the population. Especially to the parents of these girls, who will be informed about the vaccine’s characteristics. Another step in which the health sector will participate is in obtaining informed consent and prescription.
It will be administered in schools and vaccination centers in health areas designated for this purpose. International studies show that the vaccine can be administered on the same day as another vaccine in the country’s schedule.
The only thing that should be avoided is the use of immunoglobulins in conjunction with its administration or blood products in the three months prior to vaccination. Because they decrease the possibility of generating antibodies.
CECOLIN uses recombinant technology, which does not contain viral DNA. The person does not have the virus, is highly immunogenic, safe, effective, and its only contraindication is a history of hypersensitivity.
The inoculation will be a 0.5 milliliter dose administered intramuscularly in the arm. Reactions may include mild pain, mild swelling, and fever in the first 24 hours. But with very mild symptoms. One-hour monitoring at the vaccination center is recommended.
It is the family physician’s responsibility to monitor symptoms 24 hours a day, 72 hours a day, seven days a day, and 15 days after vaccination, she concluded. Before the immunogen was approved on December 30th, 2019, the Chinese National Medical Products Administration approved the vaccine. It was officially launched in May 2020.
The WHO deemed the vaccine safe and prequalified it for use in 2021. On March 12th, 2023, the results of a Phase I study were published. Concluding that the CECOLIN vaccine candidate was preliminarily shown to be well-tolerated and immunogenic. Later, on October 4th, 2024, the WHO prequalified CECOLIN for use in a single-dose regimen. Therefore, it is a well-tolerated vaccine that produces antibodies against the virus.
Its protection and effectiveness against serotypes 16 and 18 of persistent HPV infection. Which account for approximately 70 percent of all infections, have been demonstrated. This virus is also responsible for a high percentage of cervical cancer cases. One of the leading causes of death in women globally.
With the introduction of this vaccine in the National Immunization Program. Also the prevention strategy is strengthened and the onset of cervical cancer cases in younger generations is more than a decade earlier. This brings the total number of vaccines included in the program to 14, eight of which are domestically produced.
- The 4th edition of the Master’s Program in Natural Medicine will begin in Holguin - 16 de January de 2026
- Blood Bank Inaugurated at Holguin Military Hospital - 15 de January de 2026
- Holguin Cancer Center Plans to Incorporate Endoscopic Surgery in Urology - 14 de January de 2026