Aging doesn’t always imply the death of neurons. Often, what occurs is a loss of efficiency in synaptic connections, those tiny structures that allow brain cells to communicate. With age, brain plasticity decreases, and difficulties with remembering, learning, or maintaining attention appear. Until now, most studies have focused on irreversible damage such as that observed in Alzheimer’s. However, the latest research suggests that certain molecular changes may be reversible.
Scientists have long wondered what really drives this cognitive decline. Is it just inevitable wear and tear, or are there specific factors that accelerate it? The new study partially answers this question by identifying FTL1 (Ferritin Light Chain 1) as a pro-aging factor in the brain. It is a protein related to iron storage that, in excess, destabilizes the delicate neuronal balance.
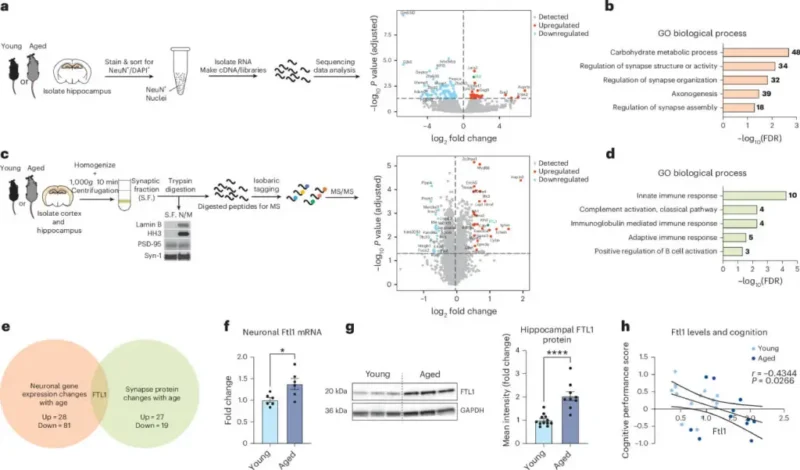
This finding, published in Nature Aging, is key because it demonstrates that brain aging is not a predetermined fate. The accumulation of FTL1 in the hippocampus, the brain region essential for memory, was directly associated with worse results on cognitive tests in aged mice. This means that a single molecular factor can make the difference between remembering and forgetting.

The hidden role of iron in memory
Iron is essential for life. It participates in energy production and multiple cellular processes. But when its regulation fails, it can become a silent enemy. In the brain, iron levels must be precisely maintained. What the researchers observed is that, with age. FTL1 accumulates excessively within neurons, altering the way iron switches between its chemical forms.
This imbalance causes an increase in oxidized iron (Fe³⁺), which in turn impacts the energy metabolism of neurons and the health of synapses.
In experiments, young mice forced to produce more FTL1 showed premature signs of brain aging: weaker synapses, less branched neurons, and memory lapses.
The effect was so clear that the animals stopped preferring a new object over a familiar one, a classic memory test. They also lost the ability to navigate a maze correctly, something that young, healthy mice solve without difficulty. The conclusion is overwhelming: too much FTL1 turns a young brain into an old brain.
Blocking the Protein to Rejuvenate
The logical question was: what happens if FTL1 is removed or blocked in an already aged brain? The researchers performed the opposite experiment. Reducing the protein’s presence in the hippocampus of aged mice. The results were surprising: the neurons recovered some of their connections and the animals’ memory improved.
Tests showed that, after treatment, the mice were able to recognize new objects and remember routes through a maze more efficiently.
At the cellular level, an increase in excitatory and inhibitory synapses was observed. That is, the communication highways between neurons became stronger and more active.
This rejuvenation was not a mere mirage. It was also confirmed in molecular analyses: by reducing FTL1. The neurons recovered receptors and proteins essential for synaptic plasticity. In other words, the brains of the aged mice began to behave like those of younger animals.
Energy, metabolism, and memory
The study went beyond superficial observations, but delved into deeper mechanisms. By comparing the genetic activity of neurons, changes were detected in key metabolic pathways. Particularly in the production of ATP, the molecule that acts as the energy currency in cells.
When FTL1 increased, ATP production plummeted, leaving neurons without enough energy to maintain their complex functions. In contrast, by blocking the protein, genes related to cellular respiration and energy synthesis were reactivated. This discovery reinforces the idea that brain aging is largely linked to energy failures in neurons.
To confirm this link, the researchers tested a supplement: NADH, a coenzyme that boosts ATP production. When administered to mice with excess FTL1, they were able to counteract some of the negative effects, restoring memory and synaptic health. The evidence is clear: improving metabolism can reverse cognitive decline associated with aging.
Beyond mice: What does it mean for us?
Although the work was conducted in animal models, its relevance transcends. In humans, high ferritin levels and iron metabolism disorders are known to be associated with poorer cognitive performance and an increased risk of Alzheimer’s. In fact, mutations in the Ftl1 gene cause a rare neurodegenerative disease called neuroferritinopathy, characterized by motor and memory problems.
The results in mice suggest that the same mechanism could be at play in human aging. The accumulation of FTL1 in the brain could act as a silent trigger for cognitive decline.
And most promising: as a specific and well-identified protein. It could become a therapeutic target for developing drugs that slow or reverse memory loss.
Scientists insist that there is still a long way to go before these findings can be translated into humans. But the simple fact of demonstrating that blocking FTL1 rejuvenates the brain of an older animal opens up a whole new horizon in aging research.
A Future for Memory
The discovery of FTL1 as a key protein in brain aging adds to a paradigm shift in science: the idea that the aging brain can be rejuvenated. It is no longer simply a matter of accepting deterioration as inevitable. But rather of searching for the molecular keys that allow it to be delayed or even reversed.
This work, conducted by an international team led by the University of California, San Francisco, not only identifies FTL1 as a silent enemy of memory, but also demonstrates that acting upon it is possible. And by doing so, essential functions such as synaptic plasticity and the ability to learn are revitalized.
For millions of people concerned about cognitive decline, the results offer a dose of hope. Although there is still no treatment ready for humans, the research points a clear path: if we control the molecules that fuel aging, we can protect memory and the identity that defines us.
References
Remesal, L., Sucharov-Costa, J., Wu, Y. et al. Targeting iron-associated protein Ftl1 in the brain of older mice improves age-related cognitive impairment. Nat Aging (2025). doi: 10.1038/s43587-025-00940-z
Translated by Aliani Rojas Fernandez
- The Challenge of Cuban Tourism - 15 de December de 2025
- Díaz-Canel Calls for Addressing the People’s Real Problems - 15 de December de 2025
- Climate Study Implemented in Holguin - 15 de December de 2025